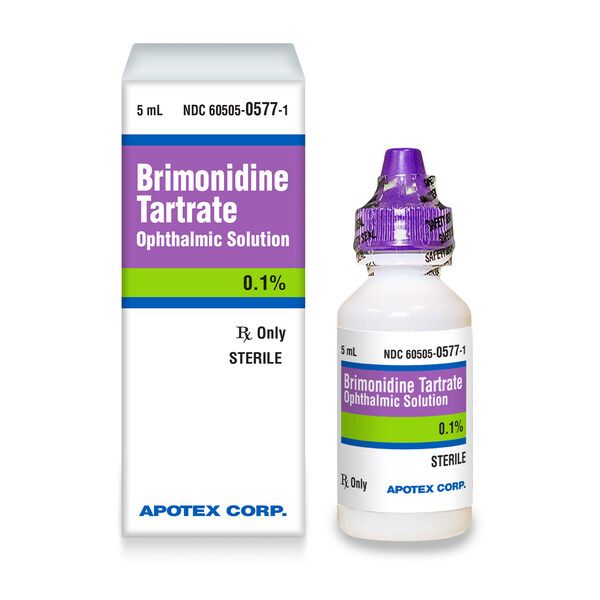
Eye Drop Recall Apotex . key facts of the apotex eye drop recall: apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some. the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march 1 due to cracks in the caps. apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or.
from www.ophthalmologytimes.com
Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march 1 due to cracks in the caps. on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. key facts of the apotex eye drop recall: another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some. The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for.
Apotex Corp. announces the launch of brimonidine tartrate ophthalmic
Eye Drop Recall Apotex key facts of the apotex eye drop recall: the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march 1 due to cracks in the caps. another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. key facts of the apotex eye drop recall: apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some.
From tahneecathy.blogspot.com
Eye drop recall TahneeCathy Eye Drop Recall Apotex the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. key facts of the apotex eye drop recall: apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some. another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. Initiated a. Eye Drop Recall Apotex.
From glance.eyesoneyecare.com
Nationwide recall issued for two more eye drop products Eye Drop Recall Apotex apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. Initiated a voluntary recall for. Eye Drop Recall Apotex.
From felicityblake.pages.dev
Eyedrop Recall 2025 Felicity Blake Eye Drop Recall Apotex on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. apotex, a canadian pharmaceutical company, recalled prescription eyedrops. Eye Drop Recall Apotex.
From decambiar.com
Se han retirado las marcas de gotas para los ojos 2023 y los números de Eye Drop Recall Apotex another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. key facts of the apotex eye drop recall: The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. Initiated a. Eye Drop Recall Apotex.
From www.ophthalmologytimes.com
Apotex Corp. announces the launch of brimonidine tartrate ophthalmic Eye Drop Recall Apotex The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. another manufacturer, apotex, on march 1 recalled six. Eye Drop Recall Apotex.
From cecna-io.ghost.io
Recent Recalls Purely Soothing & Apotex Eye Drops, Tesla Model Y Eye Drop Recall Apotex apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some. another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. the fda posted recall notices for certain. Eye Drop Recall Apotex.
From iaijos.jumpingcrab.com
Recalled eye drop brands 2023 and Apotex and Pharmedica lot numbers Eye Drop Recall Apotex key facts of the apotex eye drop recall: apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march. Eye Drop Recall Apotex.
From consumer.healthday.com
Two More Brands of Eye Drops Recalled Over Infection Risks Consumer Eye Drop Recall Apotex key facts of the apotex eye drop recall: The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march 1 due to cracks in the caps. the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over.. Eye Drop Recall Apotex.
From myparistexas.com
Third eye drop brand recalled, one linked to serious infection, vision Eye Drop Recall Apotex another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. key facts of the apotex eye drop recall: The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level. Eye Drop Recall Apotex.
From www.expertinstitute.com
FDA Recall of Eye Drops EzriCare, Alphagan P, Purely Soothing Eye Drop Recall Apotex on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march 1 due to cracks in the caps. key. Eye Drop Recall Apotex.
From www.everydayhealth.com
FDA Recall for Eye Drops From Walmart, Target, CVS, Rite Aid Eye Drop Recall Apotex apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march 1 due to cracks in the caps. on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. . Eye Drop Recall Apotex.
From www.cnn.com
Bacteria in recalled eye drops linked to cases of vision loss, surgical Eye Drop Recall Apotex on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. apotex, a canadian pharmaceutical company, recalled prescription eyedrops. Eye Drop Recall Apotex.
From www.articledashboard.com
Eye Drops Recalled Over Serious Infection Risk Eye Drop Recall Apotex key facts of the apotex eye drop recall: apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. Initiated a voluntary recall for six lots of brimonidine tartrate ophthalmic solution on march 1 due to cracks in the caps. on march 1, apotex recalled prescription eyedrops used to. Eye Drop Recall Apotex.
From www.cbs8.com
Eye drop recall More deaths, injuries linked to bacteria Eye Drop Recall Apotex the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including an over. key facts of the apotex eye drop recall: on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some. The recalled eye. Eye Drop Recall Apotex.
From www.sportskeeda.com
Eye Drops Recall 2022 Everything to know about Pharmasave and Eye Drop Recall Apotex on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. key facts of the apotex eye drop recall: another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. apotex corp., with the knowledge of the us fda, is initiating a voluntary. Eye Drop Recall Apotex.
From www.consumernotice.org
Apotex Eye Drops Uses, Dosage, Side Effects & More Eye Drop Recall Apotex apotex corp., with the knowledge of the us fda, is initiating a voluntary recall at the consumer level for. on march 1, apotex recalled prescription eyedrops used to reduce eye pressure in people with glaucoma or. another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. apotex, a. Eye Drop Recall Apotex.
From www.youtube.com
Eye Drop Recall Linked to Infections and Vision Loss YouTube Eye Drop Recall Apotex another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. key facts of the apotex eye drop recall: The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some. Initiated a voluntary recall for six. Eye Drop Recall Apotex.
From www.ophthalmologytimes.com
Apotex Corp. issues voluntary nationwide recall of brimonidine tartrate Eye Drop Recall Apotex apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some. another manufacturer, apotex, on march 1 recalled six lots of its own brand of glaucoma drops, called brimonidine. The recalled eye drops are brimonidine tartrate ophthalmic solution 0.15% and require a. the fda posted recall notices for certain eyedrops distributed by pharmedica and apotex, including. Eye Drop Recall Apotex.